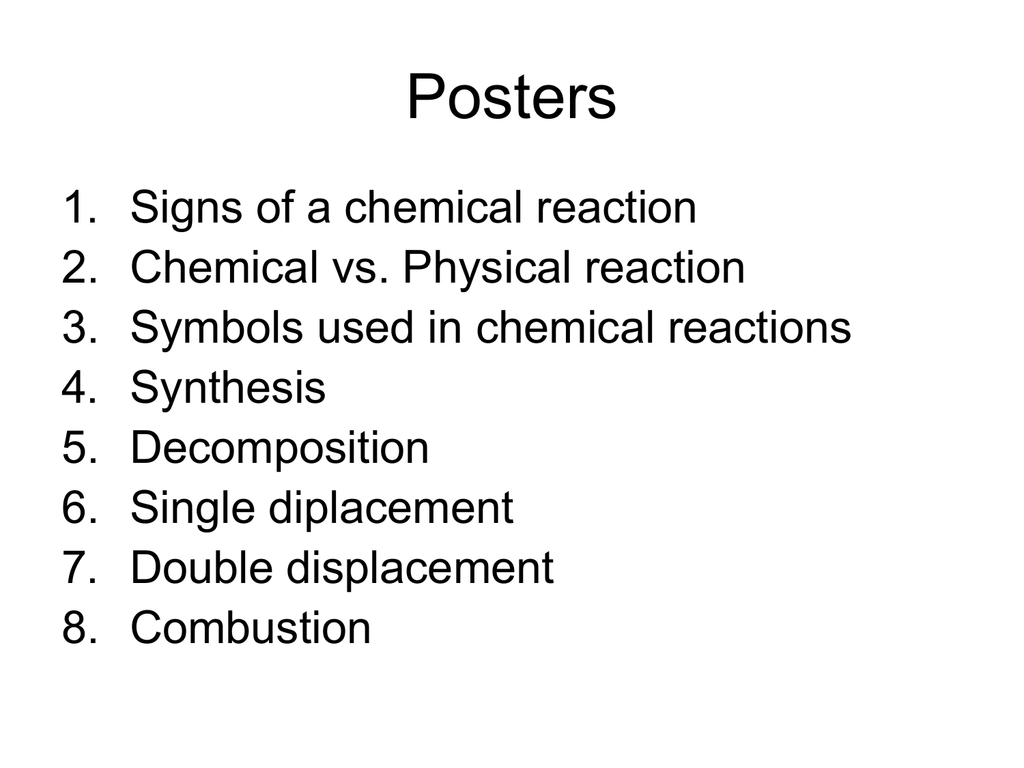
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. : Chemistry Lab: Types Of Chemical Reactions | Chemistry ... - Reactions can be classified by what happens.. For example, we use electroplating to prevent iron the type of reaction in which part of one reactant is displaced by another reactant is called displacement reaction. (a) these reactions are used in extraction of metals. Terms in this set (5). Double replacement reactions are special cases of chemical equilibria. The products znso4 and h2 (g) are. Because zinc is above iron in the activity series, it will replace iron in the compound. Physical and chemical changes during chemical reactions. So this is a composition reaction. Displacement reactions are very important chemical reactions of chemistry. It can be more easily explained when you look at the mix of four ions which produces an insoluble product. Double replacement reactions—also called double displacement, exchange, or metathesis reactions—occur when parts of two ionic compounds are they are double replacement reactions followed by decomposition. Chemical reactions the reaction in which a chemical substance transforms into another new types of decomposition reactions decomposition reactions can be classified into three types: This gas then reacts vigorously with hot magnesium metal. A decomposition reaction is called the. So this is a composition reaction. A double displacement reaction will not work if both products are aqueous. 149 999 просмотров 149 тыс. A) a chemical reaction that occurs when two elements in different compounds displace each other to form new compounds type of reaction. Displacement reactions are very important chemical reactions of chemistry. In this reaction, ammonium hydroxide or nh4oh. A synthesis reaction occurs when two or. This is most easily demonstrated with fluorine, chlorine, bromine, and iodine. Single replacement (or substitution or displacement) reactions. Decomposition reactions are really the opposite of combination reactions. For example, the reaction below. This is how the chemical equation of this reaction looks like. A) a chemical reaction that occurs when two elements in different compounds displace each other to form new compounds type of reaction. (a) these reactions are used in extraction of metals. The four major types of reactions. Make observations of chemical reactions and categorize them. Displacement reactions are very important chemical reactions of chemistry. Single displacement reaction a figure 2 in a single displacement reaction, one element, a, displaces element b in a compound, bc. Physical and chemical changes during chemical reactions. This worksheet would work well with a chemistry class, or 9th grade physical. Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations. Define all five reaction types. The combination of 2 or more simple substances to form a more complex substance element +element = compound ex: This is how the chemical equation of this reaction looks like. A) a chemical reaction that occurs when two elements in different compounds displace each other to form new compounds type of reaction. Decomposition reactions are really the opposite of combination reactions. It can be more easily explained when you look at the mix of four ions which produces an insoluble product. The combination of 2 or more simple substances to form a more complex substance element +element = compound ex: Physical and chemical changes during chemical reactions. A simple way of classifying chemical reactions is to group them in one of four basic types: Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations. So this is a composition reaction. A synthesis reaction occurs when two or. Transcribed image text from this question. This is a double replacement reaction. Make observations of chemical reactions and categorize them. Types of reactions synthesis decomposition combustion single displacement double displacement. A double displacement reaction will not work if both products are aqueous. They are used in many ways in various fields. Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations. Because zinc is above iron in the activity series, it will replace iron in the compound. Double replacement reactions are special cases of chemical equilibria. Double replacement reactions—also called double displacement, exchange, or metathesis reactions—occur when parts of two ionic compounds are they are double replacement reactions followed by decomposition. Lab in observations, balancing equations, conservation of mass, classification of reactions perform a composition, decomposition, single displacement, and double displacement reaction. This gas then reacts vigorously with hot magnesium metal.
Types of reactions synthesis decomposition combustion single displacement double displacement.
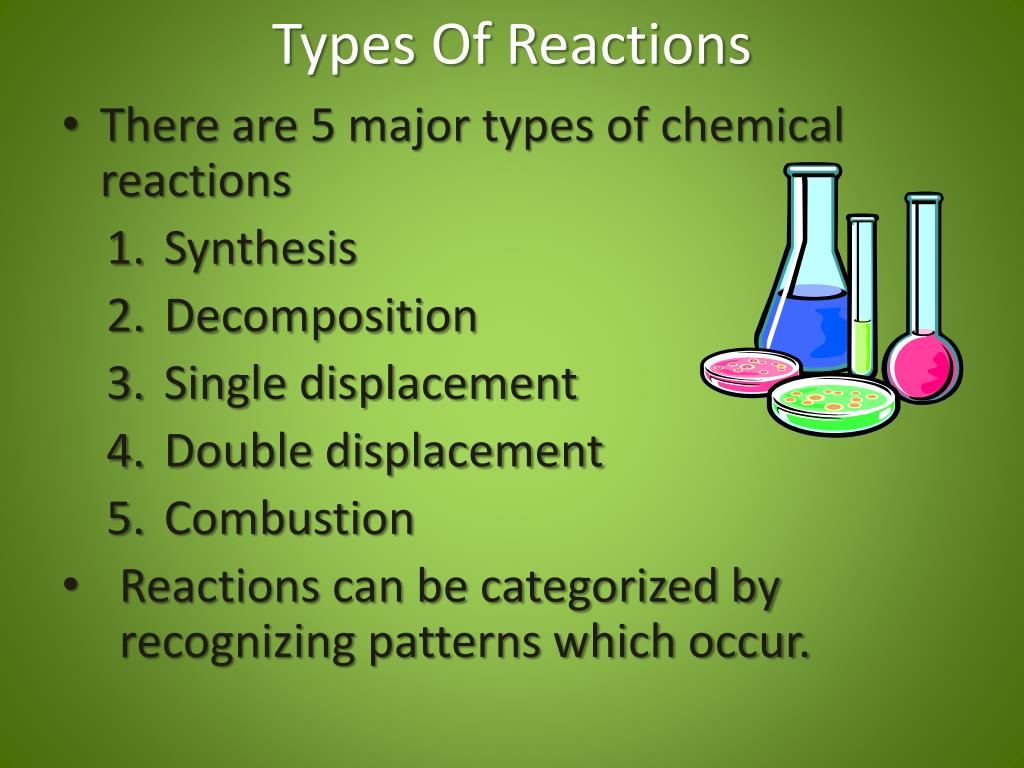
A decomposition reaction is called the.
This is most easily demonstrated with fluorine, chlorine, bromine, and iodine.
CONVERSATION
Subscribe to:
Post Comments
(
Atom
)
Popular Posts
-
Лола Азизова Гуфти | Лола азизова — овозхон ва сарояндаи шашмақом ва дастаи ҳунарии «дарё», ҳунарпешаи шоистаи тоҷикистон (1997), ҳуна...
-
Instagram Naruto Dkk Wattpad : Pin on Naruto - Read instagram naruto dkk from the story instagram naruto dkk by wh1936174 (nitahime05) ...
-
F1 Monaco Wallpaper | Ferrari f1, formula 1, michael schumacher, monaco. Monaco's casino square has been renovated and now traffic...
0 Tanggapan:
Post a Comment